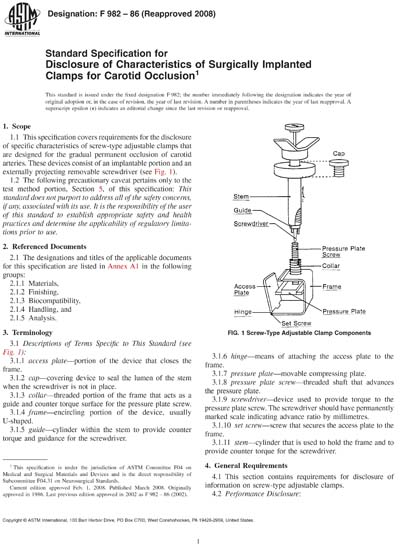
Historical
ASTM F982-86(2008)
Standard Specification for Disclosure of Characteristics of Surgically Implanted Clamps for Carotid Occlusion
1.1 This specification covers requirements for the disclosure of specific characteristics of screw-type adjustable clamps that are designed for the gradual permanent occlusion of carotid arteries. These devices consist of an implantable portion and an externally projecting removable screwdriver (see Fig. 1).
1.2 The following precautionary caveat pertains only to the test method portion, Section 5, of this specification: This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
Content Provider
ASTM International [astm]